Advanced Multiplexing for Spectral Confocal Microscopy
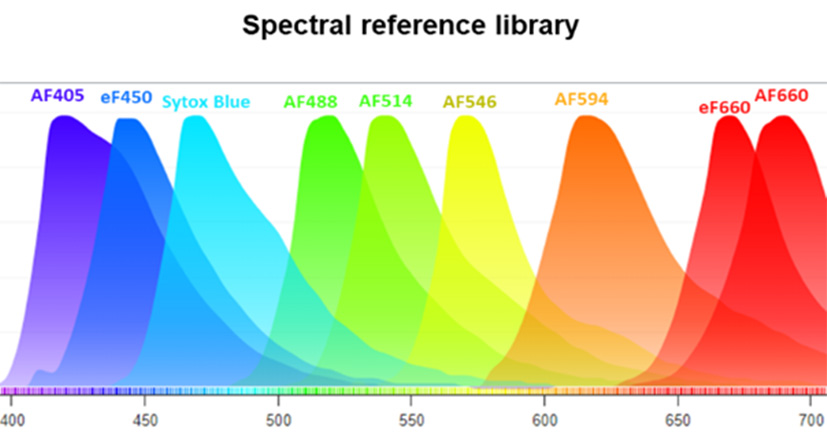
The CIML platform has successfully enhanced the spectral confocal microscopy parameters, expanding from 10 (Ref 1) to over 14. Current high-plex imaging techniques allow up to 40 colors to be captured, though they present limitations with extended acquisition times due to iterative imaging cycles, and are predominantly used in 2D imaging. To overcome these challenges, we leverage near-infrared detectors (Zeiss LSM 980 NIR) and test new-generation dyes with large Stokes shifts in sequential laser spectral acquisition mode. In immunology, this innovation enables the identification of a wide array of immune cell populations within a single tissue section, with confocal-level resolution and expedited acquisition times.
Applications
Advanced multiplexing for spectral confocal microscopy
The CIML platform has successfully enhanced the spectral confocal microscopy parameters, expanding from 10 (Ref 1) to over 14.
Current high-plex imaging techniques allow up to 40 colors to be captured, though they present limitations with extended acquisition times due to iterative imaging cycles, and are predominantly used in 2D imaging.
To overcome these challenges, we leverage near-infrared detectors (Zeiss LSM 980 NIR) and test new-generation dyes with large Stokes shifts in sequential laser spectral acquisition mode. In immunology, this innovation enables the identification of a wide array of immune cell populations within a single tissue section, with confocal-level resolution and expedited acquisition times.
Advancements in Flow Cytometry for Immune Profiling
Historically, immunology has greatly benefited from flow cytometry techniques, particularly Fluorescence Activated Cell Sorting (FACS).
These techniques allow in-depth analysis of immune cell populations within a system characterized by cell circulation across different sites in the body, such as primary and secondary lymphoid organs, sites of inflammation, infection, or tumorization.
Recent technological advancements in spectral cytometry have expanded the capacity to characterize up to 40 markers simultaneously, providing a comprehensive view of the immune system’s diverse cell types and pathophysiological states.
However, these techniques require tissue dissociation, disrupting the natural cell architecture and intercellular relationships critical for immune responses.
Bridging Flow Cytometry and Histology in 3D Contextual Imaging
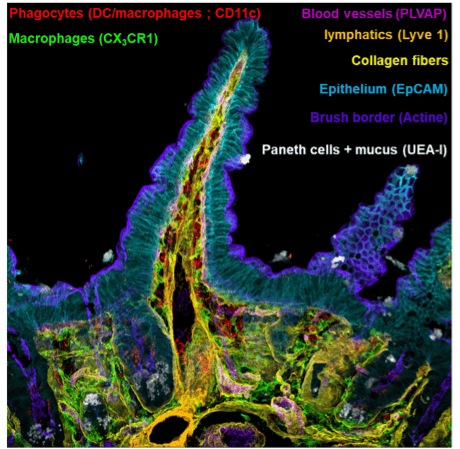
To complement flow cytometry, there is a growing need for techniques that preserve tissue architecture, enabling the study of spatial interactions between immune cells, such as phagocytes and lymphocytes, within 3D structures.
Confocal imaging with extended infrared spectral range addresses this gap, offering the capability to identify optimal fluorochrome sets for detailed acquisitions and validating standardized data analysis protocols (Ref 2).
These advancements bring new possibilities to immunology, combining flow cytometry’s high-dimensional cellular profiling with the contextual insights provided by histological imaging.
References:
(1) H. Lelouard, S. Mailfert, M. Fallet. A ten-color spectral imaging strategy to reveal localization of gut immune cell subsets. Application note (zeiss.fr)
(2) E. Germani, H. Lelouard, M. Fallet. SAPHIR: a Shiny application to analyze tissue section images. F1000Research, Faculty of 1000, 9, 1276-1285 (2020) DOI: 10.12688/f1000research.27062.2

Enables imaging with over 14 spectral parameters in confocal microscopy.

Near-infrared detectors provide fast, high-resolution multi-color tissue analysis.

Bridges flow cytometry and histology, preserving spatial and interaction data.

Supports 3D immune cell interaction studies with standardized analysis procedures.

Advanced multi-color imaging for immune cell analysis