PICsL EXPERTISES
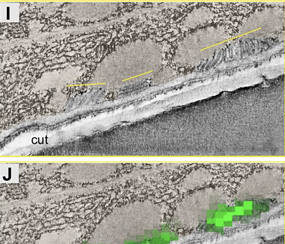
Correlative Light and Electron Microscopy (CLEM) combines the live-cell imaging capabilities of fluorescent light microscopy with the high-resolution structural details of electron microscopy.
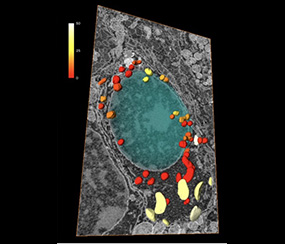
Electron microscopy usually captures life in 2D, while every biological structure is built and evolves in a 3D environment. Volume EM is a family of techniques which enables you to understand how the organelles interact and how they build together a full cell.
CryoCLEM microscopy combines cryo-electron microscopy (CryoEM) with cryo-light microscopy, enabling the correlation of molecular localization with high-resolution structural details in frozen samples.
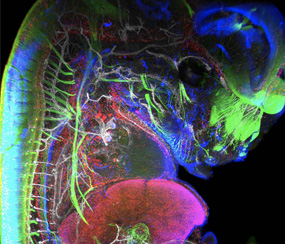
Light sheet microscopy, often referred to as single plane illumination microscopy (SPIM), is a rapidly emerging technology that combines optical sectioning with multiple-view imaging to observe tissues and living organisms with impressive resolution.
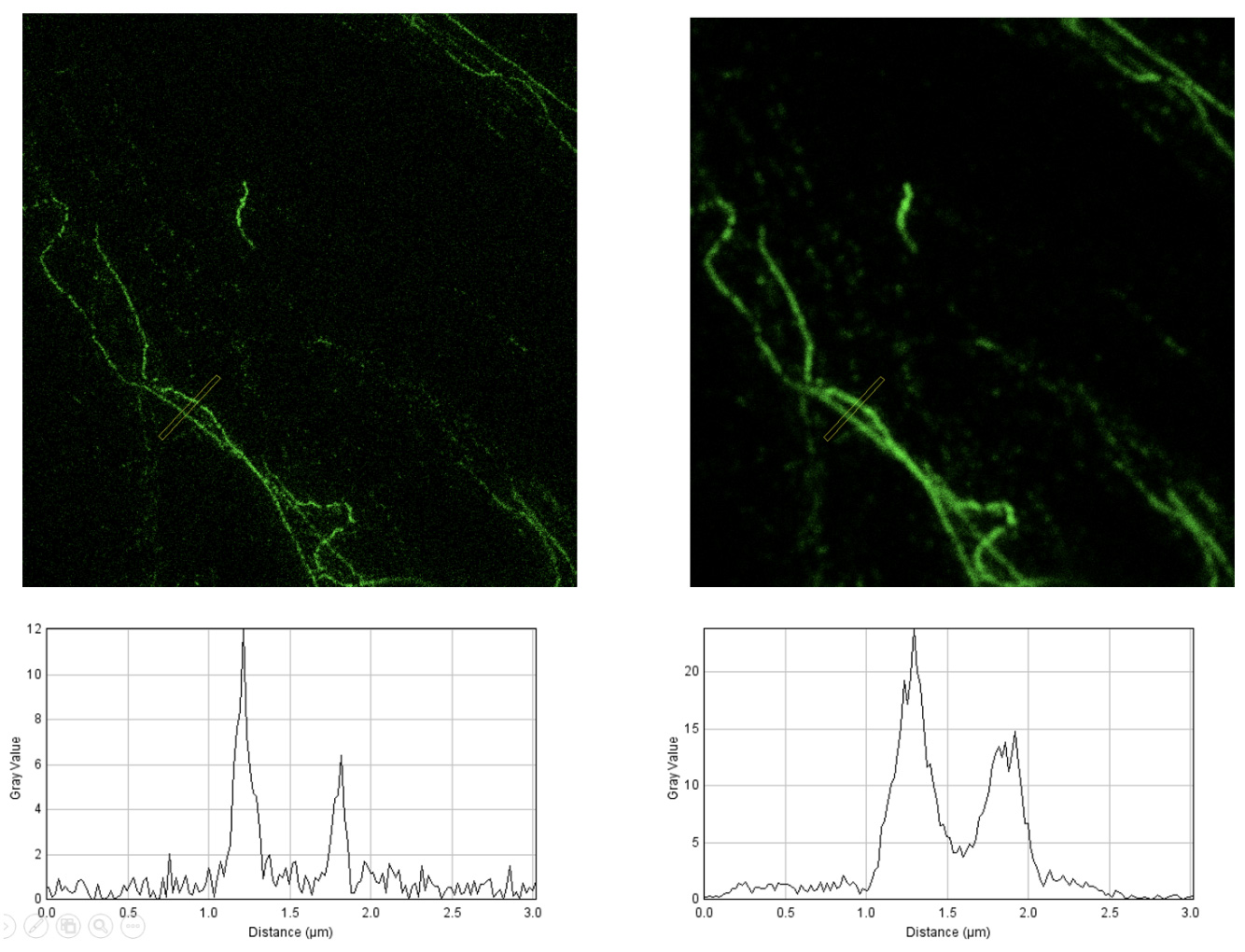
The development of super-resolved fluorescence microscopy, recognized by the 2014 Nobel Prize in Chemistry awarded to Eric Betzig, Stefan W. Hell, and William E. Moerner, revolutionized light microscopy by overcoming the diffraction limit.
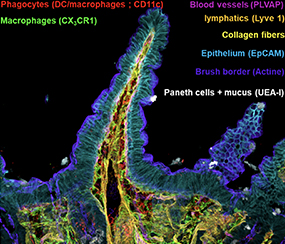
In order to quantify a large number of cell populations in tissues with 3D confocal resolution, we are developing spectral acquisition strategies enabling us to visualize 12 to 14 markers simultaneously.
In vivo imaging is based on the use of dedicated pulsed lasers that induce non-linear effects, enabling images and films to be acquired at depth (up to 1 mm) without damaging the tissue and with little background noise
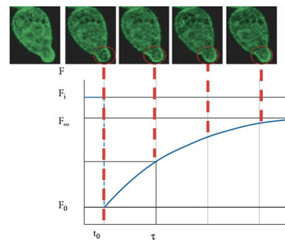
Functional Imaging combines advanced microscopy techniques to study molecular interactions, diffusion, and cellular dynamics with exceptional precision.
New-generation instruments can be used to create custom imaging acquisition sequences with a pipeline of functions including deep-learning methods that can adapt acquisition to past or future events.
Multidimentional image analysis
Lightsheet microscopy and confocal imaging of thick tissues generate complex 3D images that are often difficult or impossible to analyze using classical software such as ImageJ or QuPath.
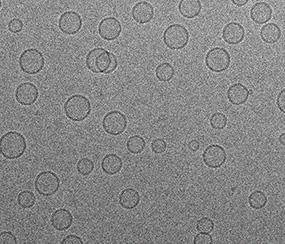
Cryo-electron microscopy (CryoEM) is the technique that captures high-resolution images of frozen molecules, preserving their natural structure. It’s crucial for studying proteins, viruses, and molecular interactions.
Correlative Light and Electron Microscopy (CLEM) combines the live-cell imaging capabilities of fluorescent light microscopy with the high-resolution structural details of electron microscopy.
Electron microscopy usually captures life in 2D, while every biological structure is built and evolves in a 3D environment. Volume EM is a family of techniques which enables you to understand how the organelles interact and how they build together a full cell.
CryoCLEM microscopy combines cryo-electron microscopy (CryoEM) with cryo-light microscopy, enabling the correlation of molecular localization with high-resolution structural details in frozen samples.
Light sheet microscopy, often referred to as single plane illumination microscopy (SPIM), is a rapidly emerging technology that combines optical sectioning with multiple-view imaging to observe tissues and living organisms with impressive resolution.
The development of super-resolved fluorescence microscopy, recognized by the 2014 Nobel Prize in Chemistry awarded to Eric Betzig, Stefan W. Hell, and William E. Moerner, revolutionized optical microscopy by overcoming the diffraction limit.
In order to quantify a large number of cell populations in tissues with 3D confocal resolution, we are developing spectral acquisition strategies enabling us to visualize 12 to 14 markers simultaneously.
In vivo imaging is based on the use of dedicated pulsed lasers that induce non-linear effects, enabling images and films to be acquired at depth (up to 1 mm) without damaging the tissue and with little background noise.
Functional Imaging combines advanced microscopy techniques to study molecular interactions, diffusion, and cellular dynamics with exceptional precision.
New-generation instruments can be used to create custom imaging acquisition sequences with a pipeline of functions including deep-learning methods that can adapt acquisition to past or future events.
Multidimentional image analysis
Lightsheet microscopy and confocal imaging of thick tissues generate complex 3D images that are often difficult or impossible to analyze using classical software such as ImageJ or QuPath.
Cryo-electron microscopy (CryoEM) is the technique that captures high-resolution images of frozen molecules, preserving their natural structure. It’s crucial for studying proteins, viruses, and molecular interactions.